RESEARCH
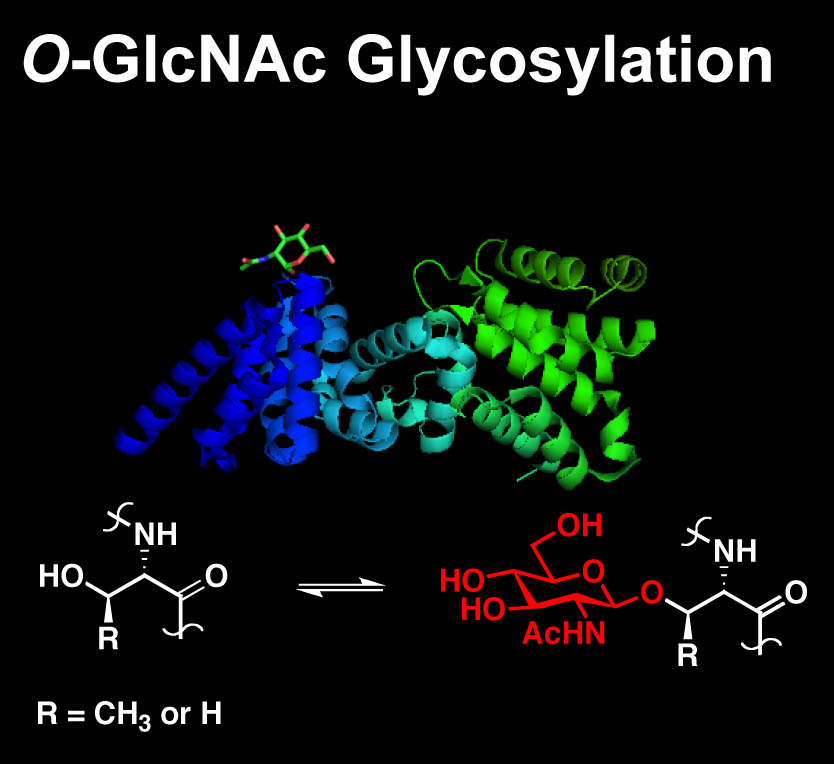
O-GlcNAc Glycosylation
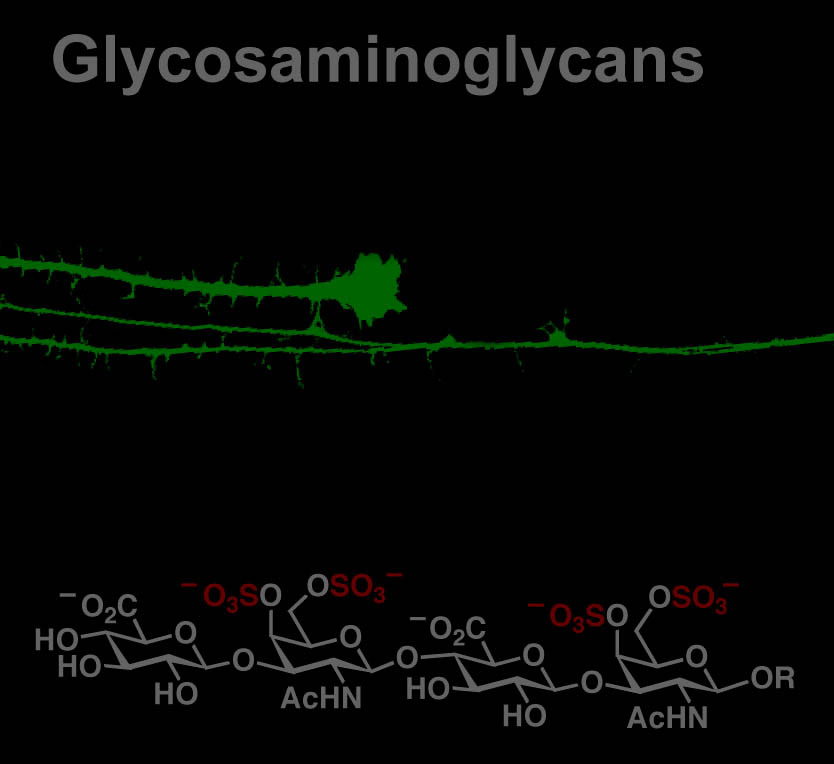
The chemical or posttranslational modification of proteins has been shown to be a fundamental mechanism by which information is transmitted and stored within the cell. We are studying O-GlcNAc glycosylation, the covalent attachment of N-acetylglucosamine (GlcNAc) to serine and threonine residues of proteins. This intracellular, reversible form of glycosylation shares many features with protein phosphorylation, a fundamental mechanism for communication in the brain. In addition, O-GlcNAc is essential for cell survival and plays important roles in many biological processes (e.g., transcription, translation, cell division) and human diseases (e.g., diabetes, Alzheimer’s disease, cancer).
Our laboratory has focused on understanding the functional roles of O-GlcNAc glycosylation in the brain. First, we are developing new chemical tools to accelerate the detection and study of O-GlcNAc glycosylation in cells and tissues. Second, we are exploring how O-GlcNAc glycosylation affects the structure and function of neuronal proteins, with a focus on proteins important for gene expression, neuronal communication and synaptic plasticity.
We have developed a chemoenzymatic strategy to selectively label O-GlcNAc-modified proteins with fluorescent or biotin tags. These chemical tools have enabled the first proteomic studies of O-GlcNAc glycosylation, revealing over 200 O-GlcNAc-modified proteins from the mammalian brain. Our chemical approaches also facilitate rapid and sensitive detection of O-GlcNAc, cellular imaging of glycosylated proteins, quantification of O-GlcNAc dynamics in cells and mapping of the glycosylation sites. Overall, our studies indicate that O-GlcNAc glycosylation is dynamically induced by neuronal activity and is abundant on proteins involved in transcription, neuronal signaling, learning and memory.