|
|
|
|
||
|
|
|
|||
|
|
|
|||
|
|
The Bercaw Group is investigating the general area of organotransition metal chemistry. New compounds are prepared using specialized vacuum line, Schlenk, and glove box techniques, and they are commonly characterized by multinuclear NMR spectrometry and by single crystal X-ray diffraction methods. Reaction mechanisms are studied by isotopic labeling, characterization of intermediates, dynamic NMR techniques, and monitoring of reaction kinetics and stereochemistry. These studies are ultimately directed toward assessing the roles of transition metals in catalysis and developing new stoichiometric and catalytic reactions for converting readily available molecules such as olefins and alkanes into more valuable products.
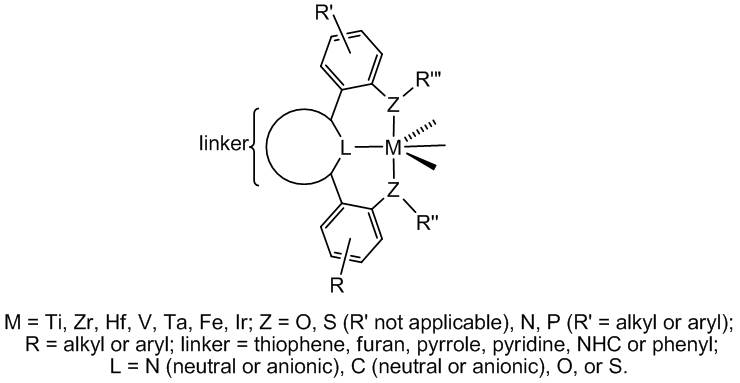
Metal Complexes Using New Tridentate Pincer Ligands
We are interested in the effect of using pincer ligands with semi-rigid aryl-aryl linkages. Such ligands form six-membered rings upon coordination to a metal center, which may engender unique, non-planar geometries for such complexes. Control of catalyst geometry in early transition metal catalyzed olefin polymerization is an important concern industrially, especially as it relates to polymer tacticity. We are also utilizing this ligand archetype as a scaffold for middle and late transition metal organometallic chemistry.
Selected references:
1. Amine, Amido, and Imido Complexes of Tantalum Supported by a Pyridine-Linked Bis(phenolate) Pincer Ligand: Ta-N Pi-Bonding Influences Pincer Ligand Geometry. Tonks, I. A.; Henling, L. M.; Day, M. W.; Bercaw, John E. Inorg. Chem. 2009, 48, 5096-5105.
2. Synthesis and Characterization of Iron Derivatives Having a Pyridine-Linked Bis(anilide) Pincer Ligand. Weintrob, E. C.; Tofan, D.; Bercaw, J. E. Inorg. Chem. 2009, 48, 3808-3813.
3. Synthesis and Reactivity of Tantalum Complexes Supported by Bidentate X2 and Tridentate LX2 Ligands with Two Phenolates Linked to Pyridine, Thiophene, Furan, and Benzene Connectors: Mechanistic Studies of the Formation of a Tantalum Benzylidene and Insertion Chemistry for Tantalum-Carbon Bonds. Agapie, T.; Day, M. W.; Bercaw, J. E. Organometallics 2008, 27, 6123-6142.
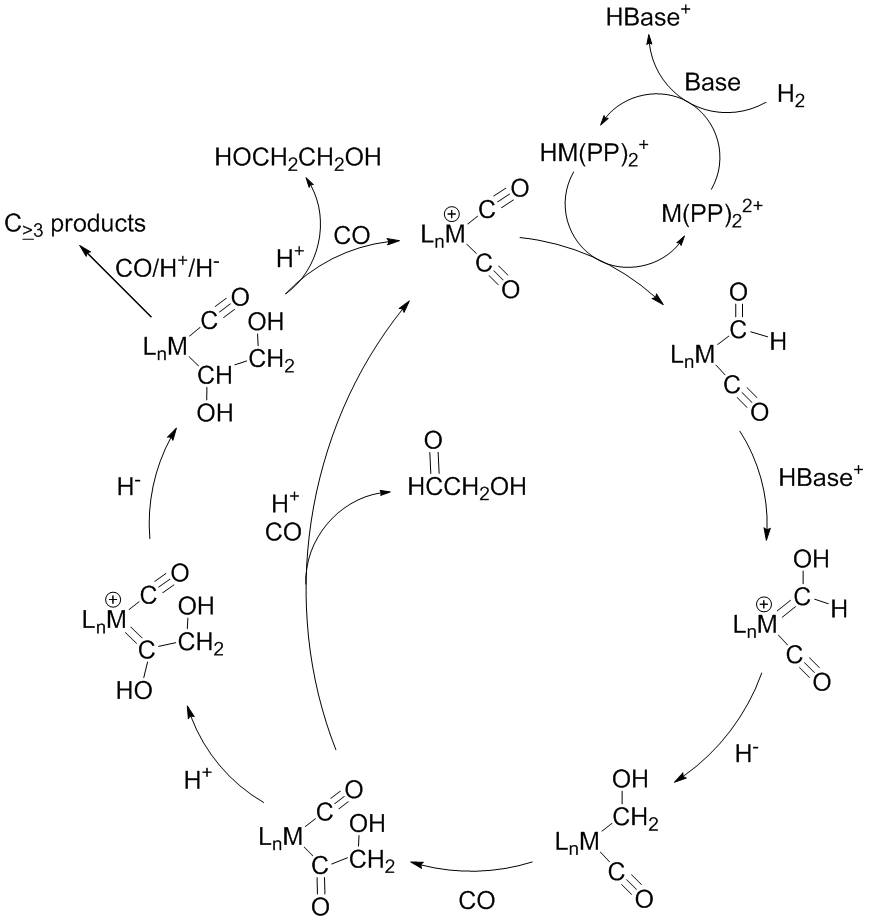
Synthesis Gas Chemistry
We are exploring new catalytic systems capable of converting synthesis gas (CO + H2) to produce useful Cn≥2 products. We are currently focusing on group 7 carbonyl complexes that can accept proton and hydride equivalents, obtained by heterolytically cleaving H2. Specifically, a sufficiently electrophilic carbonyl may be attacked by hydride, generating a metal formyl. Subsequently, the formyl may be protonated then coupled to another C1 fragment, to generate a C2 product. Our ultimate goal is that proton and hydride equivalents will be derived from dihydrogen. With respect to that goal, we have also carried out investigations concerning H2 activation, as well as metal hydride acidity and hydricity studies. Finally, we are examining the C-C coupling of two C1 fragments on group 10 metals.
Selected reference:
1. Reductive Coupling of Carbon Monoxide in a Rhenium Carbonyl Complex with Pendant Lewis Acids. Miller, A. J. M.; Labinger, J. A.; Bercaw, J. E. J. Am. Chem. Soc. 2008, 130, 11874-11875.
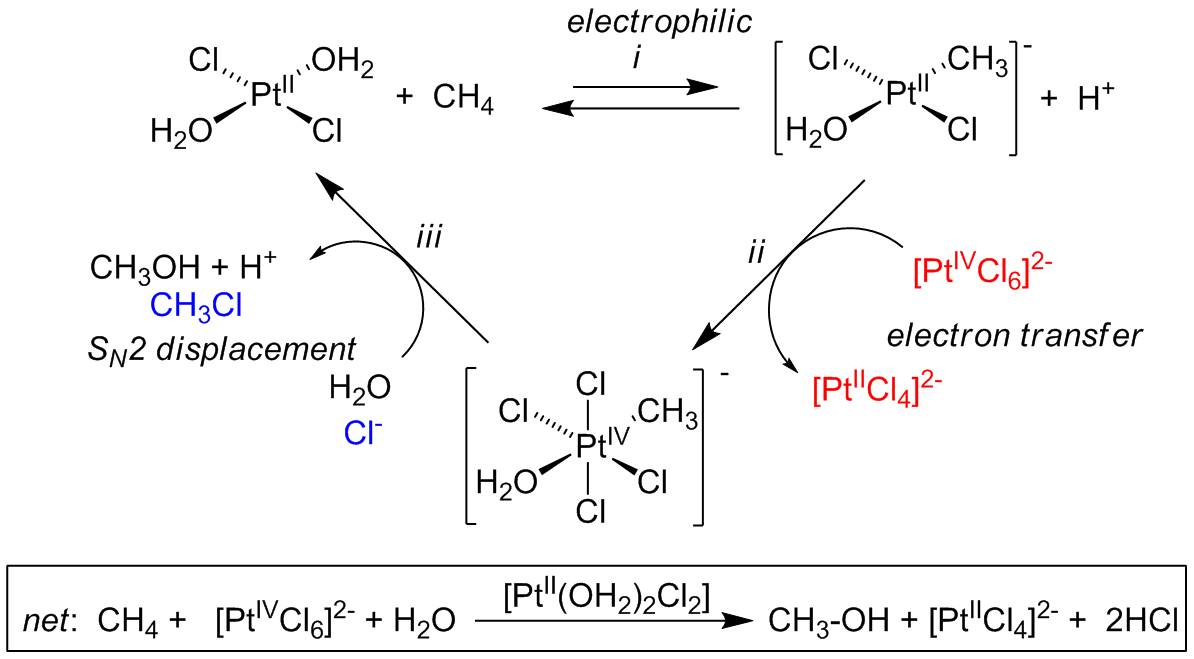
Late Metal C-H Activation
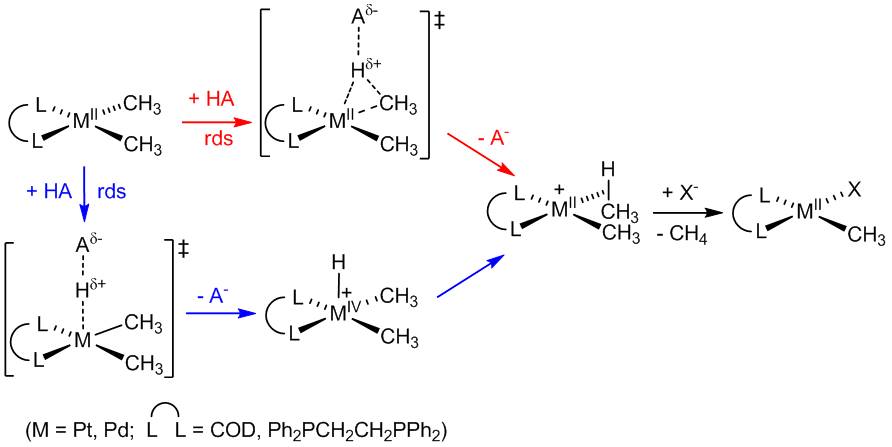
The activation of alkanes by organometallic species has attracted a lot of interest, because many examples of rapid reactions even at low temperatures have been found, with selectivity patterns quite different from those observed in free-radical reactions. We are investigating a promising organometallic system, one based on aqueous platinum halides originally reported by Shilov, that appears to escape the common constraints of incompatibility with oxidants and prohibitively large metal-oxygen bond strengths. We are pursuing the synthesis of robust electrophilic platinum(II) complexes capable of C–H activation reactions.
We are interested in studying the mechanism of the C–H activation step. In particular, we are investigating the influence of the steric and electronic factors on the reactivity and selectivity of electrophilic C–H activation in a-diimine-ligated platinum(II) complexes. We have recently begun quantitative examination of quantum-tunneling upon protonation of palladium and platinum alkyls to gain further insight into the mechanism of these reactions. To gain mechanistic understanding of the oxidation step, we have undertaken flash-quench studies to oxidize platinum II complexes, in collaboration with the Gray group.
Recently, we have begun investigating similar alkane activation using gold carbene complexes.
Selected references:
1. The role of alkane coordination in C-H bond cleavage at a Pt(II) center. Chen, G. S.; Labinger, J. A.; Bercaw, J. E. Proc. Nat Acad. Sci. USA, 2007, 104, 6915-6920.
2. Understanding and exploiting C-H bond activation. Labinger, J. A.; Bercaw, J. E. Nature, 2002, 417, 507-514.
3. Homogeneous oxidation of alkanes by electrophilic late transition metals. Stahl, S.; Labinger, J. A.; Bercaw, J. E. Angew. Chem., Int. Ed. 1998, 37, 2181-2192.
Last updated July 19, 2009. Questions? Suggestions? Raves? E-mail Web Czar |
|
||
|
|
|
|
|
|